Common connector types used in the medical field are also used in data centers, communications equipment, industrial systems, military, aerospace design and other high-reliability, high-performance markets.

In medical device design, every component choice is important. However, specifying electronic connectors for medical technology is not always specific to this market.
When designing connectors for medical use, many factors must be considered, including FDA classification of the medical device, basic electrical requirements including voltage and current, EMC and shielding requirements, and environmental requirements to specify appropriate materials.
Ⅰ. FDA classification and applicable standards for medical device connectors
The U.S. Food and Drug Administration (FDA) classifies medical devices based on the risk of using the device. Medical devices are divided into three classes: Class I (lowest risk), Class II (higher risk), and Class III (highest risk). The higher the classification, the higher the level of regulatory control. The European Union, Canada, and other countries have similar regulations.
Additionally, the FDA has classifications for the manufacturing process itself ( FDA 21 CFR 820 ) to ensure medical quality standards.
For medical electronic equipment, IEC 60601 is a series of technical standards for the safety and effectiveness of medical electrical equipment; compliance with IEC 60601-1 is a necessary condition for new equipment to obtain FDA approval.
Formal risk management methods are now an integral part of the regulatory process. Manufacturers now more closely examine the risks of each component and material used in their devices and then document those risks for FDA inspection. ISO 14971 is the ISO standard that applies risk management to medical devices. Connectors used in FDA-classified devices must meet the corresponding requirements, even though the manufacturer of the device itself is ultimately responsible for certification and risk assessment.
Ⅱ. Medical connection cable: operation requirements
The most important requirement for connectors is the safety of patients and medical staff, followed by ease of use. Since connectors are used in hospitals and other clinical environments, they should be designed to be easily operated even with surgical gloves.
The best design is a push-pull design with an audible click to indicate successful engagement. Connectors and cables can also be color-coded to ensure they are plugged into the correct receptacles. Unique mechanical coding can guarantee that the correct connector is plugged into its correct mating connector.
In many cases, the patient connector attaches to a probe or similar single-use device, but the mating connector on the instrument itself should be rugged enough to withstand up to 10,000 mating cycles.
Because many physiological signals are very small (a typical electrocardiogram (ECG or EKG) signal has a peak amplitude of about 1 mV before amplification), it is important that the electrical characteristics remain consistent over the life of the product. Low signal levels may also require EMC protection such as shielding. The rise of portable and home devices will only increase the need for robustness and ease of use.
Ⅲ. Environmental considerations
Medical connectors must withstand a variety of medical sterilization processes, including autoclave, STERRAD®, ethylene oxide (EtO), gamma radiation, and chemical sterilization. Connectors on assemblies intended for single use are typically packaged and sterilized at the factory; however, the mating sockets on the device are reused and must be sterilized in place. These sockets are often equipped with soaking caps to protect the contacts during sterilization.
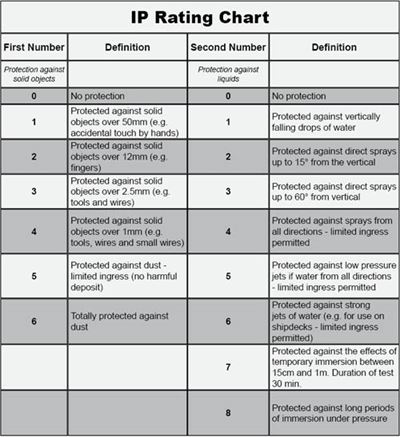
Medical interconnect systems must also be sealed against water, dust, and other potential hazards, so when paired with a matching receptacle, it should have an appropriate IP rating. Each IP number is independent; the first indicates protection against dust intrusion (levels 1 – 6), and the second indicates protection against liquid intrusion (levels 1 – 8). For example, an IP67 system has the highest level of protection against dust (6) and can withstand immersion in 1 meter of water for up to 30 minutes (7).
Ⅳ. Medical connector materials
As mentioned above, medical connectors require materials that are resistant to heat and chemicals. PPSU, PSU, and PEI are three thermoplastic high-performance plastics that have thermal, electrical, and mechanical properties suitable for medical use.
- Higher temperature limits when used in air (+180 °C continuously for PPSU 1000, +170 °C for PEI 1000 and +150 °C for PSU 1000)
- High mechanical strength, stiffness and creep resistance over a wide temperature range
- Excellent resistance to hydrolysis (suitable for repeated steam sterilization)
- High ductility even at low temperatures
- Physiologically harmless (suitable for contact with food)
- Very high dimensional stability
- Translucent, non-optical quality (except PPSU, which is black)
- Extremely resistant to high energy radiation (gamma rays and X-rays)
- Good electrical insulation properties and positive dielectric properties
Ⅴ. Trends in Medical Connectors
With the rise of portable and home medical devices, size and weight are becoming increasingly important. Miniature connectors are emerging, but the smaller size means that more attention must be paid to ISO 14971 risk management factors, such as clearance and creepage distances for high-voltage signals.
Hybrid connectors are another trend in connectors. Of course, electrical signals are critical, but many medical devices require an “umbilical cord” connection that includes fluid lines and fiber optic connections as well as wires. To ensure that all components are connected correctly, RFID tags are often another required feature. Professional manufacturers are able to meet this need by integrating multiple connectors , cables , and fluid lines into a single custom assembly . KONNRA connectors have a good reputation in the industry and have obtained multiple certifications such as ISO9001, ISO14001, ISO13485, IPC620, IATF16949, UL, etc., to ensure that the products meet strict standards in quality and environmental protection. With these certifications, KONNRA continuously optimizes the production process to improve the reliability and stability of its products, and strives to provide medical device customers with high-quality services and solutions.